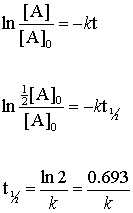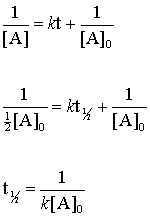half life formula for zero order reaction
K t 12 12 A 0. 3 0 3 l o g a 2 a a k t 1 2 2.

Zero Order Reaction Definition Examples Formula
If 1 lb is equal to 454 grams 1.
. For the 1 st order reaction the half-life is. It is represented by t12. T 12 A 0 2k.
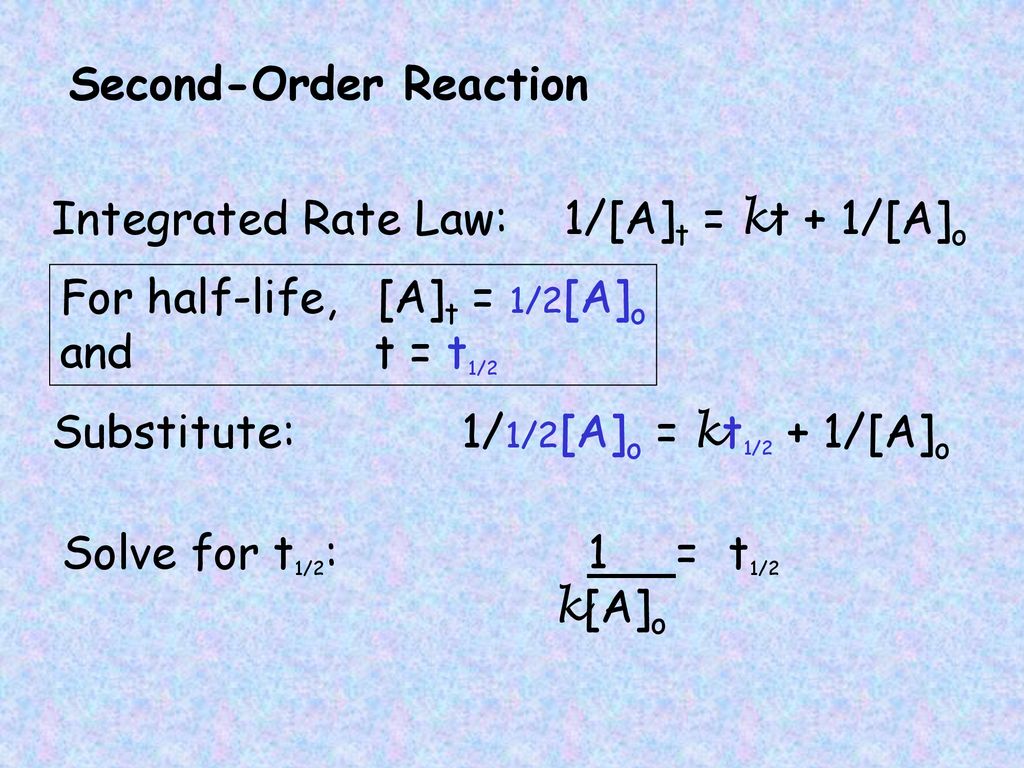
Using the concentration-time equation for a second-order reaction we can solve for half-life. For a general reaction. Here k is the rate constant A is the preexponential factor Ea is the energy of activation R is the ideal gas constant and T is absolute temperature.
The density of methane at 0 degree Celsius and 1 atm pressure is 0668gL. For a zero-order reaction the integrated rate law is. If we set the time t equal to the half-life the corresponding concentration of A at this time is equal to one-half of its initial concentration.
I Arrhenius equation is k A e E a R T or l n k l n A R T E a. The half-life of a zero-order reaction the formula is given as t12 R02k The half-life of a first-order reaction is given as t12 0693k The half-life of a second-order reaction is given by the formula 1kR0. The mathematical expression that can be employed to determine the half-life for a zero-order reaction is t 12 R 0 2k.
This is the integrated rate law for a zero-order reaction. 12 A A 0 - k t 12. The integrated rate law for the zero-order reaction A products is A_t -kt A_0.
Half-life of a first. T 12 12 k A 0. Read More Second order Reaction.
6 9 3 Thus half-life period t 1 2 of. 1A n-1 1 A 0 n-1 n-1 kt. In zero-order kinetics the rate of a reaction does not depend on the substrate concentration.
For the first-order reaction the half-life is defined as t 1. Note that this equation has the form. Half life means 50 percent of reactants disappear in that time interval.
Determine the half-life of a zero order react. NA Product The rate law of zero order kinetics is. As for other reaction orders an equation for zero-order half-life may be derived from the integrated rate law.
Thus for a first-order reaction each successive half-life is the same length of time as shown in. It is the time in which the concentration of a reactant is reduced to one half of its initial concentration. T 12 is the half-life of.
The rate constant for a zero-order reaction is 054 M-1s-1. In some cases we need to know the initial concentration A o Substitute this information into the equation for the half life of a reaction with this order and solve for t ½. However the half-life of a zero-order reaction increases as the initial concentration increases.
Derivation of half life of zero order reaction. T 12 0693k. From the above-integrated equation we have.
Half-Life of a Zero Order Reaction. A reactions half-life formula changes depending on the order of the reactions. It is to be noted that the formula for the half-life of a reaction varies with the order of the reaction.
Therefore a plot of A versus t will always yield a straight line with a slope of. As for all reaction orders the half-life for a zero-order reaction is inversely proportional to its rate constant. The half-life of a Zero-th order reaction is t A0 2kHere I derive this from the Integrated Rate LawAsk me questions.
The rate constant k for the reaction or enough information to determine it. Y m x ymx y m x. We can derive an equation for determining the half-life of a first-order reaction from the alternate form of the integrated rate law as follows.
Rate k C12H22O11 Half-Life of a reaction t12. T ½ 0693 k For a second order reaction 2A products or A B products when A B rate kA 2. Therefore A2 k 0 t ½ or t ½ A2k.
When t t ½ that is the half-life of the reaction completed the concentration of the reactant A A2. The t 1 2 formula for a zero order reaction suggests the half-life depends on the amount of initial concentration and rate constant. A A 0 - kt.
Ii The formula to calculate half life period t 1 2 of zero order reaction is t 1 2 2 k R o. Now replacing t with half-life t12 in the above equation. From the above formula the half-life of the zero order kinetics depends on.
The half-life formula for various reactions is given below. A zero order reaction implies that the rate of the reaction does not depend on the concentration of the reactant. The rate constant for the reaction can be determined from the slope of the line which is equal to -k.
The order of the reaction or enough information to determine it. It is clearly visible from the above equation that the half-life of the reaction is dependent on the rate constant as well as the initial concentration of the reactant. Half-Life of a Zero-Order Reaction The half-life of a reaction describes the time needed for half of the reactants to be.
Half life in zero order reaction. Because this equation has the form y mx b a plot of the concentration of A as a function of time yields a straight line. K-k k.
The rate constant for a zero-order reaction is. Integrated rate law for a first order reaction is k t 2. Half life formula for nth order reaction.
As for other reaction orders an equation for zero-order. 3 0 3 l o g 2 t 1 2 0. The Half-Life of Zero Order Reaction calculator computes the half-life in nuclear decay for a zero order reaction.
We know that at the half-life time eqt_12 eq the concentration of the reactant will. It is essential to note that the half-life formula of a reaction varies with the reactions order. Half life in zero order reaction.
Where A 0 Initial concentration of reactant at timet 0. 3 0 l o g a x a For half-life t t 1 2 x 2 a On substituting the values k t 1 2 2. Substituting these terms into.
6 9 3 or t 1 2 k 0.

Half Life Expressions Chemistnate

Zero Order Reactions Chemistry Class 12 Iit Jee Main Advanced Neet Aipmt Askiitians Youtube

Half Life Of Zero Th 0th Order Reaction Derivation Youtube
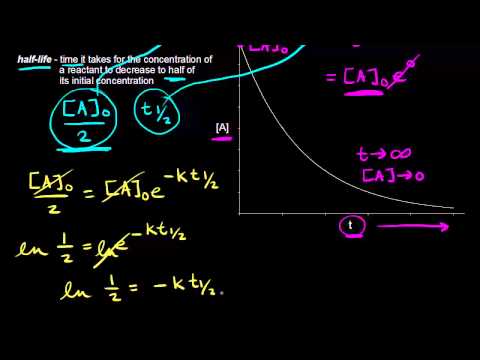
Half Life Of A First Order Reaction Video Khan Academy

Half Life Expressions Chemistnate

Derive The Integrated Half Life Equation For Zero Order Reaction Chemistry Chemical Kinetics 12889537 Meritnation Com
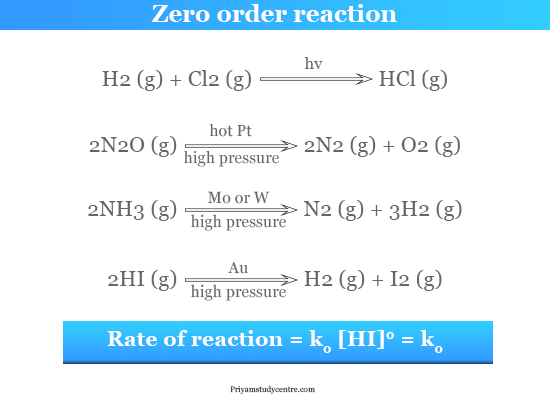
Zero Order Reaction Definition Examples Formula
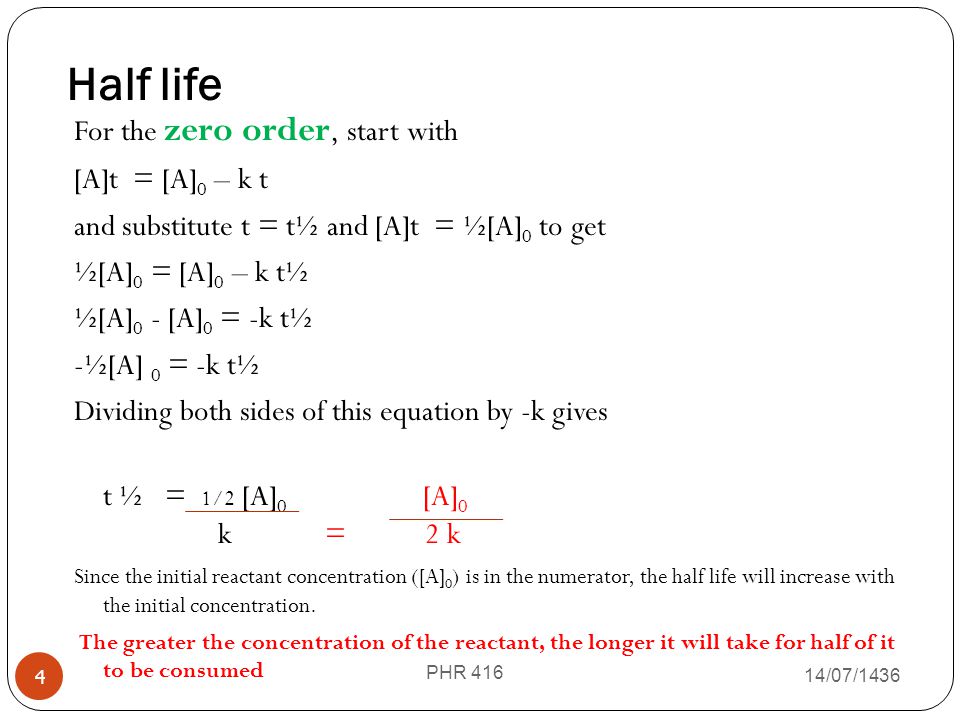
Principles And Kinetics Of Drug Stability Phr 416 Ppt Video Online Download

Half Life Expressions Chemistnate

Which Of The Following Statements Are Corrects

Zero Order Reactions Video Kinetics Khan Academy

Integrated Rate Laws Chemistry For Majors